Potassium Dichromate Colour Change
Methanol is oxidised by sodium dichromate Na 2 Cr 2 O 7 acidified in dilute sulphuric acid to form the aldehyde methanal. A leading example of pointillist technique executed on a large canvas it is a founding work of the neo-impressionist movement.

The Colour Change Of Acidified Potassium Dichromate The Real Chemist Youtube
Add 1 ml of milk in the above test tube and mix thoroughly.
. 4K 2 Cr 2 O 7 4K 2 CrO 4 2Cr 2 O 3 3O 2. Then add a few drops of 5 potassium dichromate solution. The oxidation of the alcohol to an aldehyde is indicated by the colour change of the dichromate solution as it is reduced from the orange colour of Cr 2 O 7 2 to the green of chromiumIII ions Cr 3.
As matter burns. SALT ANALYSIS SCHEME OF ANALYSIS EXPERIMENT OBSERVATION INFERENCE PRELIMINARY EXAMINATIONS. ISO GUIDE 11972 Presentation of International Standards and technical reports Withdrawn without replacement IWA 12005 Quality management systems Guidelines for process improvements in health.
Conversion of ethanol into ethanoic acid is an oxidation reaction because addition of oxygen to a substance is called oxidation. If the potassium dichromate solution changes colour from orange to green then an oxidation reaction has taken place with a primary or secondary alcohol. When the solution of K 2 Cr 2 O 7 reacts with an alkali ionic salt a yellow solution is obtained because of the potassium.
CHEMISTRY Salt analysis class 12 1. This source of. The structure of a potassium dichromate molecule is illustrated below.
In this glycerol and dichromate ions react to give a brown colour to the solution. If the contents of the test tube turn yellow then milk contains salt. The Mohr titration is sensitive to the presence of both chloride and bromide ions in solution and.
Na 2 SO 3 H 2 SO 4 Na 2 SO 4 H 2 O SO 2 The gas turns potassium dichromate paper acidified with dil. A Green to orange b Red to colorless c Orange to green d Blue to green. C The color changes from orange to green due to the formation of iron III sulphate.
You should check the result as soon as the potassium dichromateVI solution turns green - if you leave it too long the Schiffs reagent might start to change colour in the secondary alcohol case as well. Of Mn2 Pink Pre. A Sunday Afternoon on the Island of La Grande Jatte French.
Cr 2 K 2 0 7 the hexavalent form of chromium Cross-reactions. A primary alcohol will form an aldehyde and a secondary alcohol will form a ketone. Here oxygen is added to ethanol by oxidising agent like alkaline potassium permanganate or acidified potassium dichromate and it is converted into acid.
Butanol is oxidised by sodium dichromate Na 2 Cr 2 O 7 acidified in dilute sulphuric acid to form the aldehyde butanal. Aim To prepare M20 solution of Mohrs salt and. It is a good idea to first carry out a rough titration in order to become familiar with the colour change at the end point.
1 Noted the colour Colourless Absenceof Cu2 Fe2 Fe3 Mn2 Co2 Ni2 Blue Pre. Reaction to form hydrogen chromate ions or dichromate ions affecting the accuracy of the end point. Flammability The tendency of matter to burn is referred to as flammability.
Search all type Industrial Raw Materials its Sellers in India Browse by Industry Category Send Inquiry Get Multiple Sellers Responses List Raw Material Products PlasticsPolymer PharmaceuticalsDrug Chemicals Paints PesticidesInsecticide MetalsSteel OreMineral YarnFibre RubberElastomer Construction CeramicsGlass Fertilizers. There are three indicators that may be used for the titration of Fe 2 with K 2 Cr 2 O 7. It produces a cyan-blue print used for art as monochrome imagery applicable on a.
ISO 1 ISO 199. Sodium nitroprusside Complex of Purple colour 3. Change in colour of the milk to blue confirms that the milk is adulterated with Hydrogen Peroxide Or.
Of Co2 2 Noted the solubility a In water. These are diphenylamine diphenylbenzidine and diphenylamine sulfonate. H 2 SO 4 SO 2 gas is evolved which is suffocating with the smell of bur ning sulphur.
Chemical Properties of Potassium Dichromate. A monochromatic light beam is passed through this sample and a detector records the change in intensity and hence the change in color which is used to calculate the percent alcohol in the breath. Of Cu2 Brown Pre.
Certain materials are highly reactive whereas others are extremely inactive. K 2 Cr. It can be noted that crystals of potassium dichromate have a triclinic structure.
In this reaction. Take 2-3 ml of a sample in a test tube. Materials Required Apparatus Required weighing bottle weight box volumetric flask conical flask.
ISO 12016 Geometrical product specifications GPS Standard reference temperature for the specification of geometrical and dimensional properties. A typical example of this is the use of acidified potassium dichromateVI as an oxidising agent. This colour change of orange to yellow and yellow to orange can be explained on the concept of equilibrium of the two ions.
Mohrs salt Titration with Potassium Dichromate. Because of the colour change to the acidified potassium dichromateVI solution you must therefore have a secondary alcohol. However since potassium dichromate is a strong oxidizer numerous alcohol groups can be oxidized by it producing false positives.
How the colour changes when the gases after thermal decomposition of ferrous sulphate come in contact with an acidified solution of potassium dichromate. Seurats composition includes a number of Parisians at a park. Un dimanche après-midi à lÎle de la Grande Jatte was painted from 1884 to 1886 and is Georges Seurats most famous work.
Then the chromic ions oxidize the glycerol and reduce into chromous ions by giving a blue colour to the solution in the presence of nitric acid. It can be noted that in the potassium dichromate molecule potassium exhibits an oxidation state of 1 oxygen exhibits an oxidation state of -2 and chromium exhibits an oxidation state of 6. Test for Sulphite ion SO2 3 a On treating sulphite with warm dil.
In this reaction. AnsaPhysical change bChemical change cPhysical change dChemical change ePhysical change. Introducing heat to K 2 Cr 2 O 7 decomposes it into potassium chromate K 2 CrO 4 and produces O 2 gas.
Unlike permanganate dichromate titrations require an indicator. Of Ni2 Pale pink Pre. In the potassium dichromate solution Cr 2 O 7 2-ions in orange colour are in equilibrium with CrO 4 2-ions in yellow colour.
The cyanotype from Ancient Greek κυάνεος - kuáneos dark blue τύπος - túpos mark impression type is a slow-reacting economical photographic printing formulation sensitive to a limited near ultraviolet and blue light spectrum the range 300 nm to 400 nm known as UVA radiation. H 2 SO 4 green. Add 2-3 drops of Potassium Dichromate Reagent.
At work request a material safety data sheet to help identify alternatives that are safe hence avoiding contact with material containing chromates. 18Write a balanced chemical equation for each of the following reactions and also classify them. The oxidation of the alcohol to an aldehyde is indicated by the colour change of the dichromate solution as it is reduced from the orange colour of Cr 2 O 7 2 to the green of chromiumIII ions Cr 3.
The colour change for all three indicators is green to violet and the standard electrode potentials are all ca 078 V. Potassium for example is extremely reactive even in the presence of water. ALead acetate solution is treated with dilute hydrochloric acid to form lead chloride and acetic acid solution.
In practice when it is produced during a reaction in a test tube it is often green. Question 2 A mixture of oxygen and ethyne is burnt for welding. The appearance of the endpoint can be detected by the colour change of KMnO 4 from colourless to light pink.
Of Fe3 Green Pre. Cr 2 O 7 2- H 2 O 2CrO 4 2- 2H Orange-red Yellow. A pea-sized piece of potassium reacts explosively when combined with a small volume of water.
If no colour change is observed then the alcohol was a tertiary alcohol. The colour of the hexaaquachromiumIII ion has been shown as a difficult to describe violet-blue-grey in all the diagrams above. Add 3 drops of Paraphenylene Diamine and shake well.
Avoid all of these.
Content Redox Chemistry Inside The Breathalyzer The Alcohol Pharmacology Education Partnership
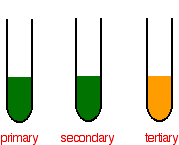
14 7 Determining Alcohol Classifications In The Lab Alternate Reactions Chemistry Libretexts
What Is The Precipitate In Potassium Dichromate Sulfuric Acid And Potassium Iodide Quora

Potassium Dichromate Test For Alcohols Stock Image C040 2657 Science Photo Library
No comments for "Potassium Dichromate Colour Change"
Post a Comment